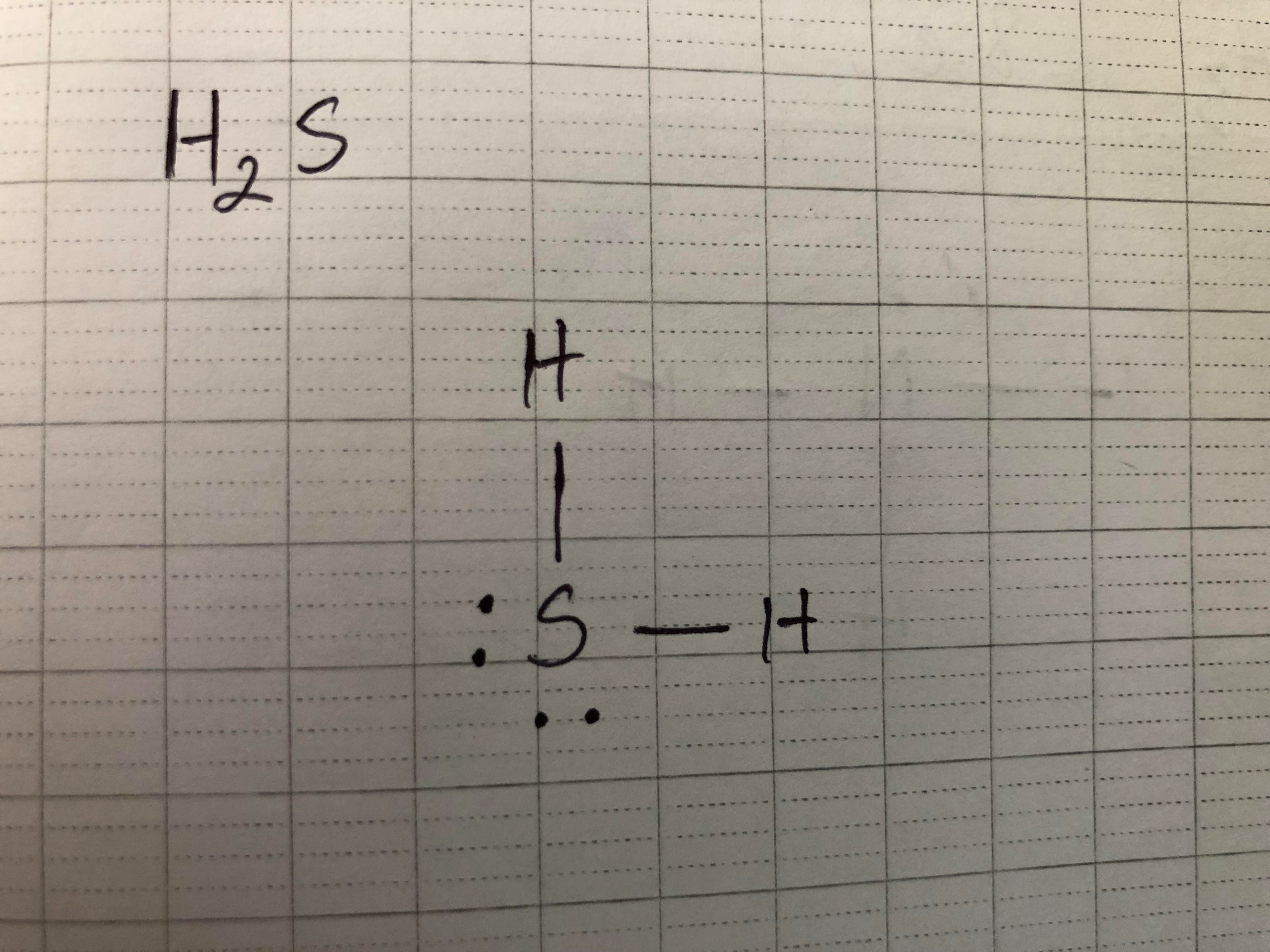Valence
Shell
Electron
Pair
Repulsion
VSEPR is a model used in Chemistry in order to predict a geometric shape of molecules based on the number of valence electrons.
We can classify molecules into 7 main geometric shapes:
- Linear 180° Non-Polar
- Bent 104.5° Polar
- Trigonal Planar 120° Non-Polar
- Trigonal Pyramidal 107.3° Polar
- Tetrahedral 109.5° Non-Polar
- Trigonal Bipyramidal 90°/ 120° Non-Polar
- Octahedral 90° Non-Polar
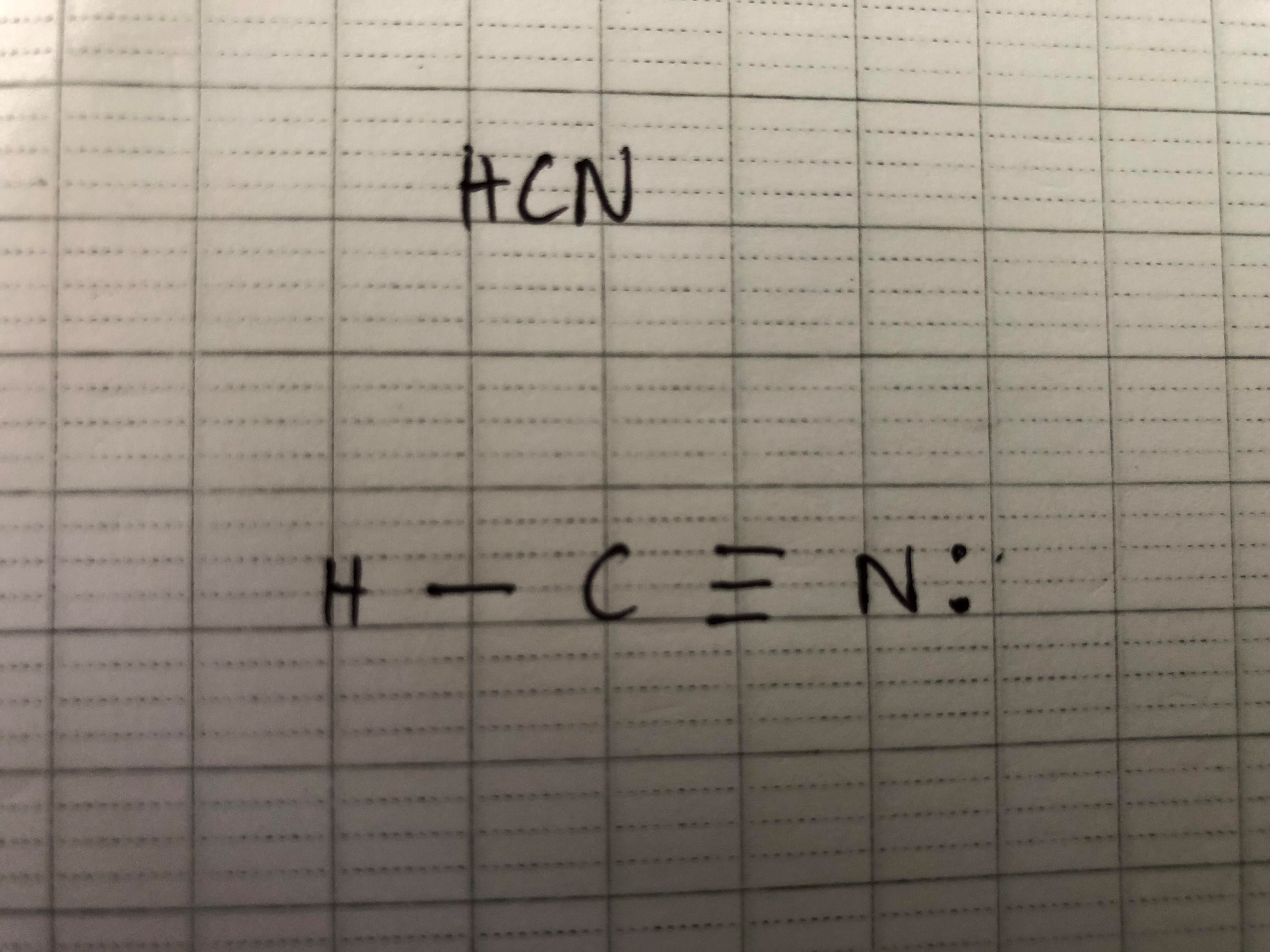
Before figuring out any of those shapes, we need to draw Lewis Dot Diagram first. Let’s look at some examples:
Based on its Lewis Dot structure, we can classify HCN as Linear.
As you can see above, Sulfur has 5 bonds. So, we would classify this as Trigonal Bipyramidal.
Now, in this case, Sulfur has only 2 bonds. We would classify this as Bent. Why? HCN also has only 2 bonds but classified as Linear. That is because there are two extra pairs of electrons in the middle atom so those two pairs of electrons will push each of the two bonds down, creating an angle.